Cryo-EM structure of the V2R:AVP:Gs complex
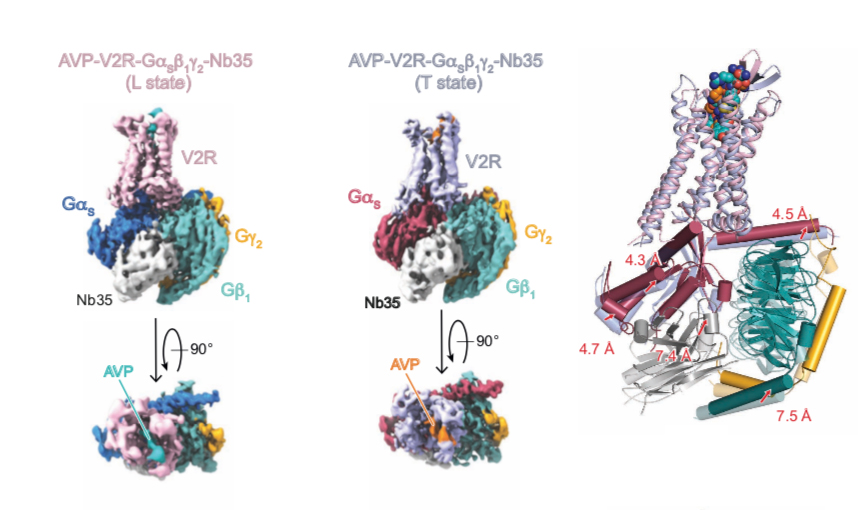
The cryo-EM structure of the antidiuretic hormone arginine-vasopressin V2 receptor signaling complex
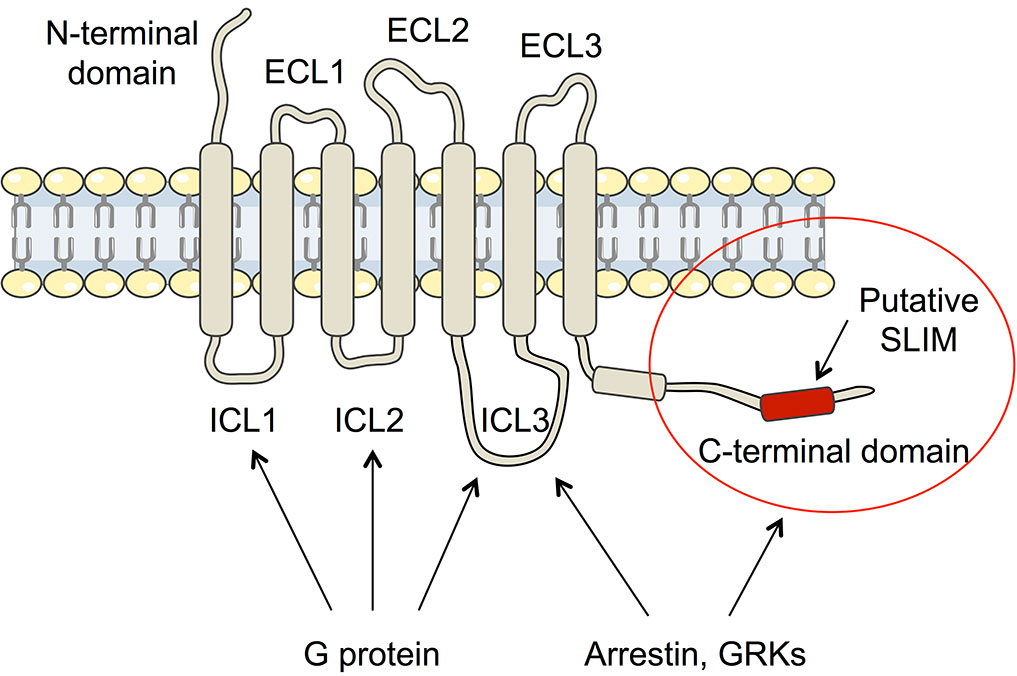
Arginine-vasopressin (AVP) is known as the antidiuretic hormone, and it regulates a vital function of our body, water homeostasis. It plays a role in the kidney at the basolateral membranes of the principal cells of the distal collecting duct of the nephron where it directly interacts with the V2 receptor subtype (V2R), which is a typical G protein-coupled receptor (RCPGs). Upon interaction, AVP activates a signaling pathway leading to fusion of aquaporine water channels to the apical membrane of the cells and consequently to water reabsorption from urine to blood. Coupling of the V2R to the Gs protein constitutes a first key step of the cellular response.
The Team « Multi-scale structural biology » in collaboration with the Team of Bernard Mouillac and Sébastien Granier from the IGF (Montpellier), determined the structure of the AVP-V2R-Gs ternary complex by cryo-electron microscopy single particle analysis in combining the cryo-EM density map with experimental NMR (Nuclear Magnetic Resonance) data obtained by Hélène Déméné (Team « Integrative Biophysics of Membranes », CBS) and computational molecular dynamic simulations developed by Nicolas Floquet at the Institut des Biomolécules Max Mousseron (IBMM).
This study reveals the extremely strong dynamics of the AVP-V2R-Gs ternary complex since three conformational states were observed reflecting different modes of interaction of AVP and the Gs protein. These structures make it possible to understand how certain mutations induce two genetics, congenital nephrogenic diabetes insipidus and the nephrogenic syndrome of inappropriate antidiuresis.
This study paves the way to the development of new therapeutic molecules able to activate or to inhibit its function. This research perspective is crucial regarding patients who are suffering for these pathologies which are difficult to manage.
Link to the article : https://advances.sciencemag.org/content/7/21/eabg5628
Cryo-electron microscopy structure of the antidiuretic hormone arginine-vasopressin V2 receptor signaling complex.
J. Bous, H. Orcel, N. Floquet, C. Leyrat, J. Lai-Kee-Him, G. Gaibelet, A. Ancelin, J. Saint-Paul, S. Trapani, M. Louet, R. Sounier, H. Déméné, S. Granier*, P. Bron*, B. Mouillac*. Science Advances, 7, eabg5628 (2021).
Dynamic stiffening of the flagellar hook
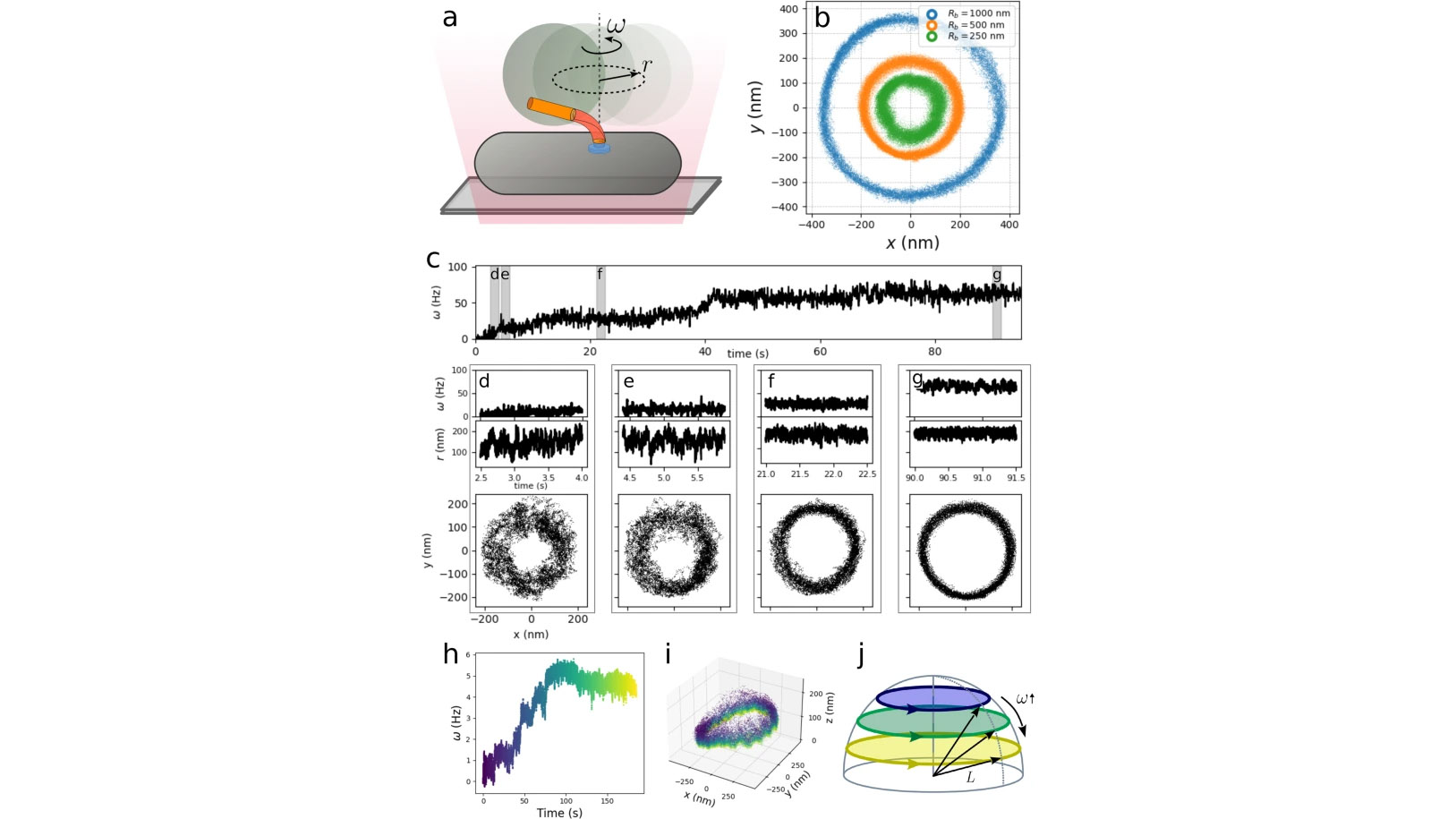
For many bacteria, motility stems from one or more flagella, each rotated by the bacterial flagellar motor, a powerful rotary molecular machine. The hook, a soft polymer at the base of each flagellum, acts as a universal joint, coupling rotation between the rigid membrane-spanning rotor and rigid flagellum. In multi-flagellated species, where thrust arises from a hydrodynamically coordinated flagellar bundle, hook flexibility is crucial, as flagella rotate significantly off-axis. However, consequently, the thrust applies a significant bending moment. Therefore, the hook must simultaneously be compliant to enable bundle formation yet rigid to withstand large hydrodynamical forces. Here, via high-resolution measurements and analysis of hook fluctuations under dynamical conditions, we elucidate how it fulfills this double functionality: the hook shows a dynamic increase in bending stiffness under increasing torsional stress. Such strain-stiffening allows the system to be flexible when needed yet reduce deformation under high loads, enabling high speed motility.
Full text: https://www.nature.com/articles/s41467-022-30295-7