Synonymous codons do not influence the amino-acid backbone conformation
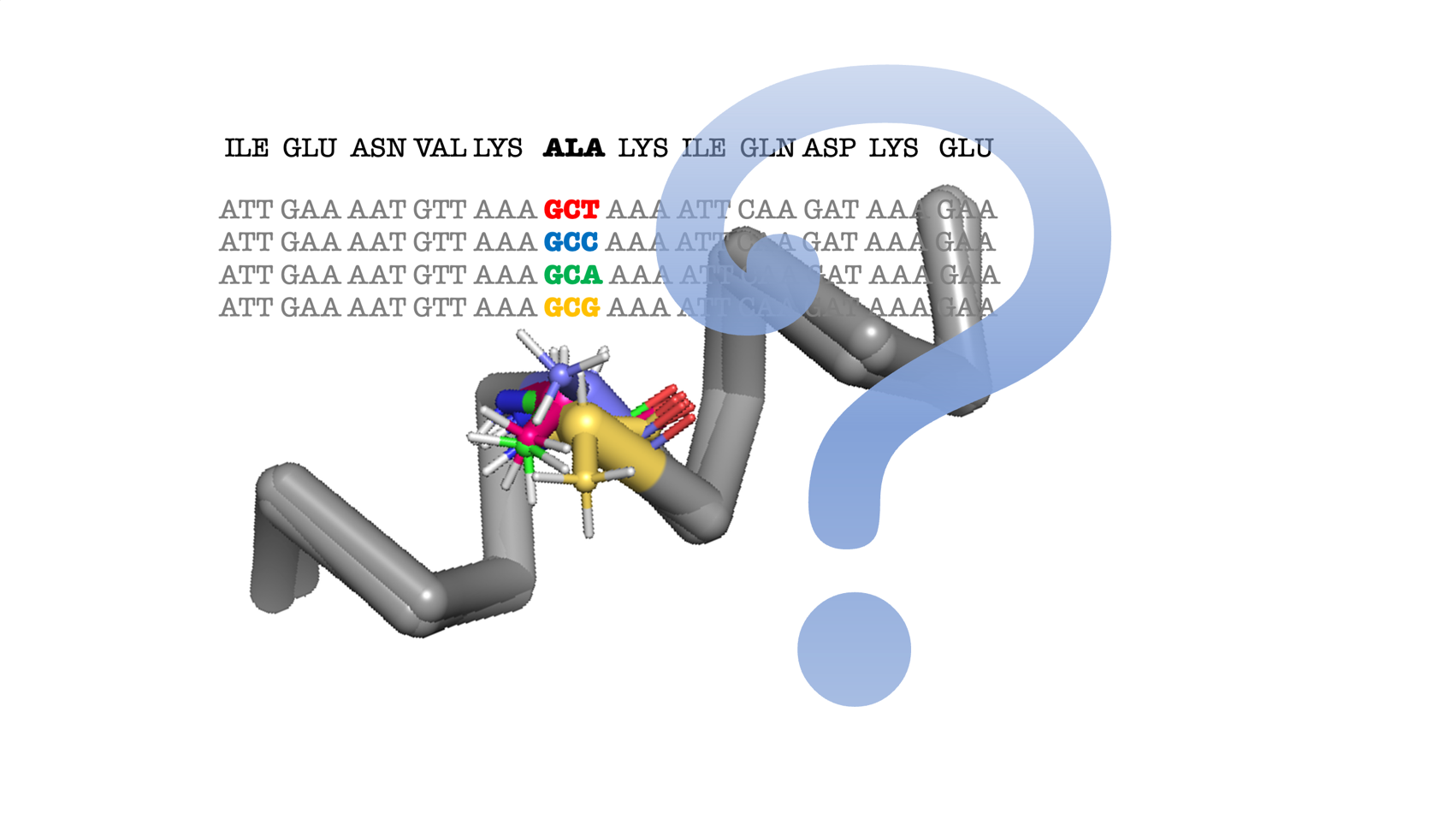
The correlation between synonymous codon usage and secondary structure in translated proteins has been widely demonstrated and plays a capital role in tuning translational rates and protein folding kinetics, indirectly influencing multiple biological processes. A recent report [A. A. Rosenberg, A. Marx, A. M. Bronstein, Nat. Commun. 13, 2815 (2022).] suggests that the translated synonymous codon influences the dihedral angles within secondary structure elements. If true, this result would have strong consequences in several scientific fields, including structural biology and protein design, where results would depend on DNA sequence rather than protein sequence. In this study, we show that the original statistical methodology used in the referred study was formally incorrect. Furthermore, when using a correct approach, we demonstrate that the influence of the codon on the distribution of the dihedral angles is not statistically significant for any type of secondary structure, reinforcing key assumptions in structural biology, protein design, and molecular evolution.
Insights into the "cocktail effect" of endocrine disruptors
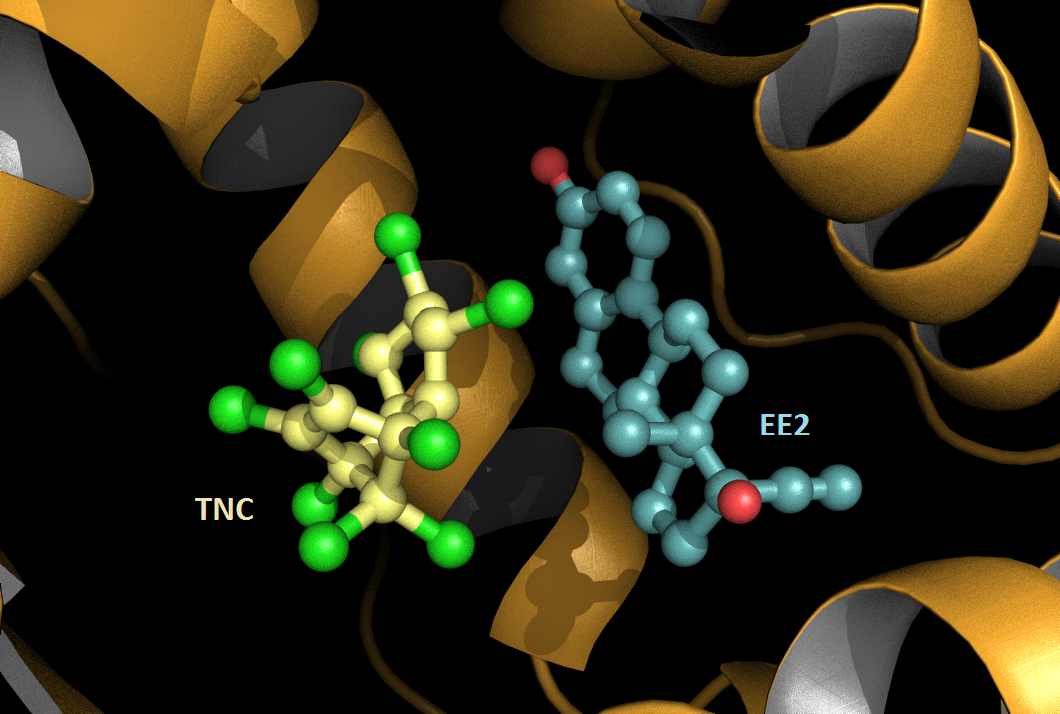
Chemicals which taken in isolation are safe for humans may become harmful when mixed . The team of William Bourguet in Structural Biochemistry Center (Inserm / CNRS / University of Montpellier), together with teams from the Cancer Research Institute (IRCM ) and the Functional Genomics Institute (IGF ) in Montpellier elucidated in vitro a molecular mechanism that may contribute to this phenomenon known as the "cocktail effect".
New publication: "Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds"
Authors: Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavaillès V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W.
Journal: Nat Communication 2015 Sep 3;6:8089.
Links: