When cryo-electron tomography uncovers the in cellulo structural organization of the Chikungunya virus replication complex
When cryo-electron tomography and sub-tomogram averaging techniques uncover the in cellulo structural organization of the Chikungunya virus replication complex within the plasma membrane

The Chikungunya virus (CHIKV) is a mosquito-borne pathogen responsible for acute musculoskeletal disease in humans. The replication of the viral RNA genome occurs within specialized membranous replication organelles (RO), also known as spherules, which house the viral replication complex. This membranous complex consists of four viral proteins (nsP1-4) along with various cellular partners.
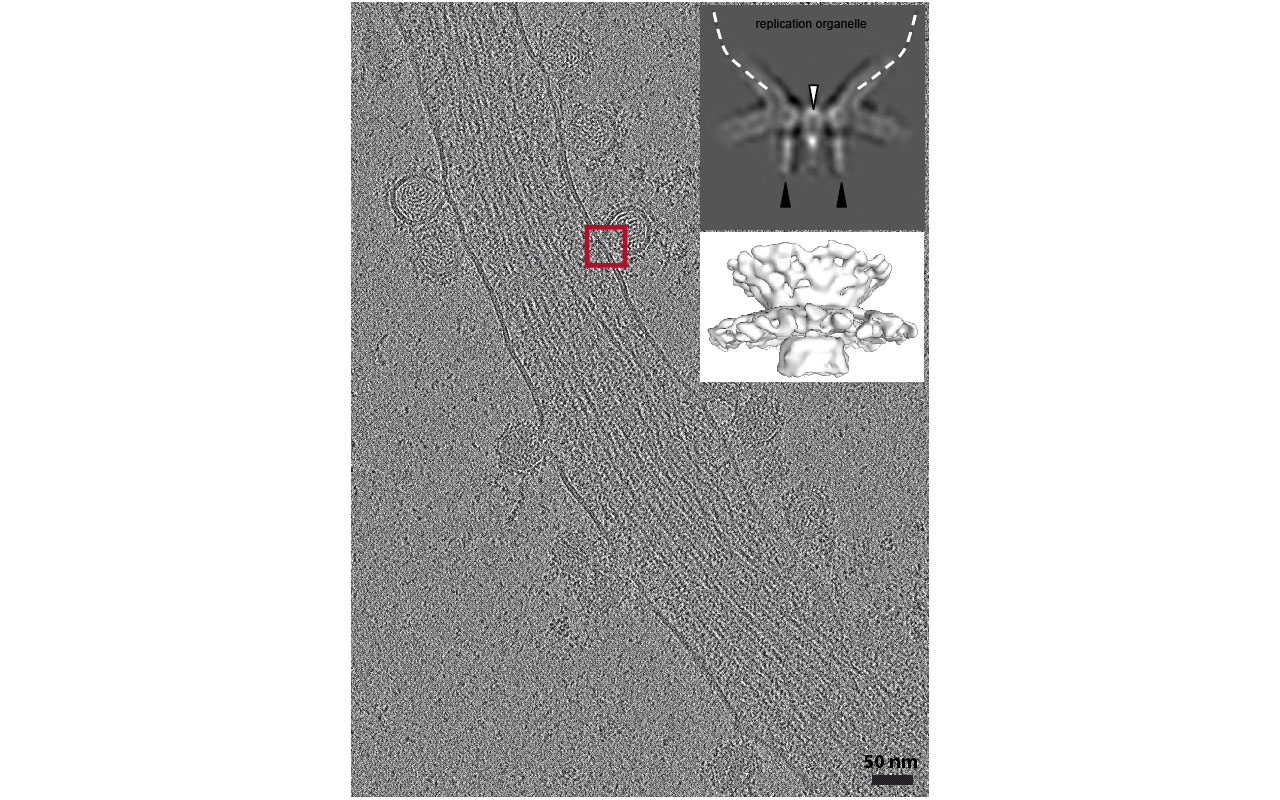
Using a combined approach of cryo-electron tomography on infected human cells at 17 hours post-infection (hpi), sub-tomogram averaging, and cryo-sectioning through CEMOVIS (Cryo-Electron Microscopy of Vitreous Sections), we investigate the structural organization of the replication complex and examine the replication dynamics within the spherules.
Collectively, this study sheds new light on the dynamic behavior of CHIKV replication organelles and the associated viral replication processes at the interface with cell membranes in infected cells.
Publication link
In situ fate of Chikungunya virus replication organelles
Justine Girard, Olivier Le Bihan, Joséphine Lai-Kee-Him, Maria Girleanu, Eric Bernard, Cedric Castellarin, Matthew Chee, Aymeric Neyret, Danièle Spehner, Xavier Holy, Anne-Laure Favier, Laurence Briant and Patrick Bron