Huntingtin: low-complexity regions at high-resolution (PI: Pau Bernadó)
People Involved: P. Bernadó, N. Sibille, F. Allemand, A. Urbanek, A. Morató, A. Fournet, M. Popovic, C. Elena
While most protein sequences are aperiodic and feature most of the 20 amino acids, many proteins harbor low complexity regions (LCRs), with a highly biased composition. LCRs are functionally re- levant and, in some cases, are directly related with severe diseases. Despite their relevance, high-resolution structural and dynamic characterization of LCRs cannot be tackled with current methods placing them on the dark side of proteome.
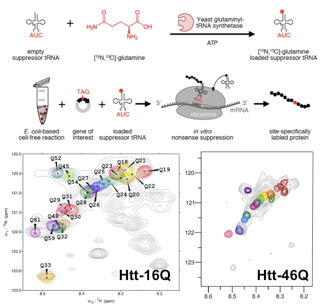
Homorepeats, a subclass of LCRs that is characterized by stretches of the same amino acid, perform very specialized functions facilitated by the localized enrichment of the same physicochemical property. In addition, numerous severe pathologies have been associated with abnormally long repetitions eg. huntingtin (Htt). The N-terminal region of Htt, known as exon-1, contains glutamine and proline stretches, and is the prototypical example of a homorepeat. The poly-Gln tract is directly linked to Huntington's disease (HD), a deadly neuropathy appearing in individuals with more than 35 consecutive Gln residues, the pathological threshold. Present structural biology approaches do not allow high-resolution studies of Htt to investigate the origin of the pathological threshold. Our group is developing chemical biology tools to enable the residue-specific isotopic labeling of Htt. This allows us for first time to study Htt at the atomic level with NMR. The applica- tion of these approaches to several Htt constructs with Q tracts of diffe- rent lenghts will shed light on the structural bases of the pathological threshold of HD.
Main collaborators: S. Delbecq (U. Montpellier), C. Cativiela (Zaragoza, Spain), J. Cortés (LAAS, Toulouse)
Grant: ANR SPIN-HD, ERC ChemRepeat