M. Cohen-Gonsaud, C. Roumestand, P. Barthe, B. Murciano, E. Guca, A. de Visch
Biologie structurale de Mycobacterium tuberculosis (MCG)
Collaboration with : G. Mukamolova (University of Leicester), P. Brodin (Institut Pasteur, Lille), V. Molle (DIMNP – Montpellier), A. Blanc-Potard (DIMNP – Montpellier).
For the next 5 years, the working group “Mtb Structural Biology” led by MCG will continue deciphering the mechanisms around the latency, phospho-regulation, persistence and virulence in M. tuberculosis. This work is also done in collaboration with the “Structures and screening of therapeutic and environmental” team (in collaboration with G. Labesse and JF Guichou) for the drug-design related project. To do so we put in place a network of collaborations with top international and national microbiology laboratories (i.e. University of Leicester, Institut Pasteur Lille), but also collaboration with le local Mtb community (University Montpellier II and CPBS).
Resuscitation and Phosphoregulation mechanisms in M.tuberculosis
Collaboration with : V. Molle (DIMNP – Montpellier), L. Kremer (DIMNP – Montpellier), G. Mukamolova (University of Leicester)
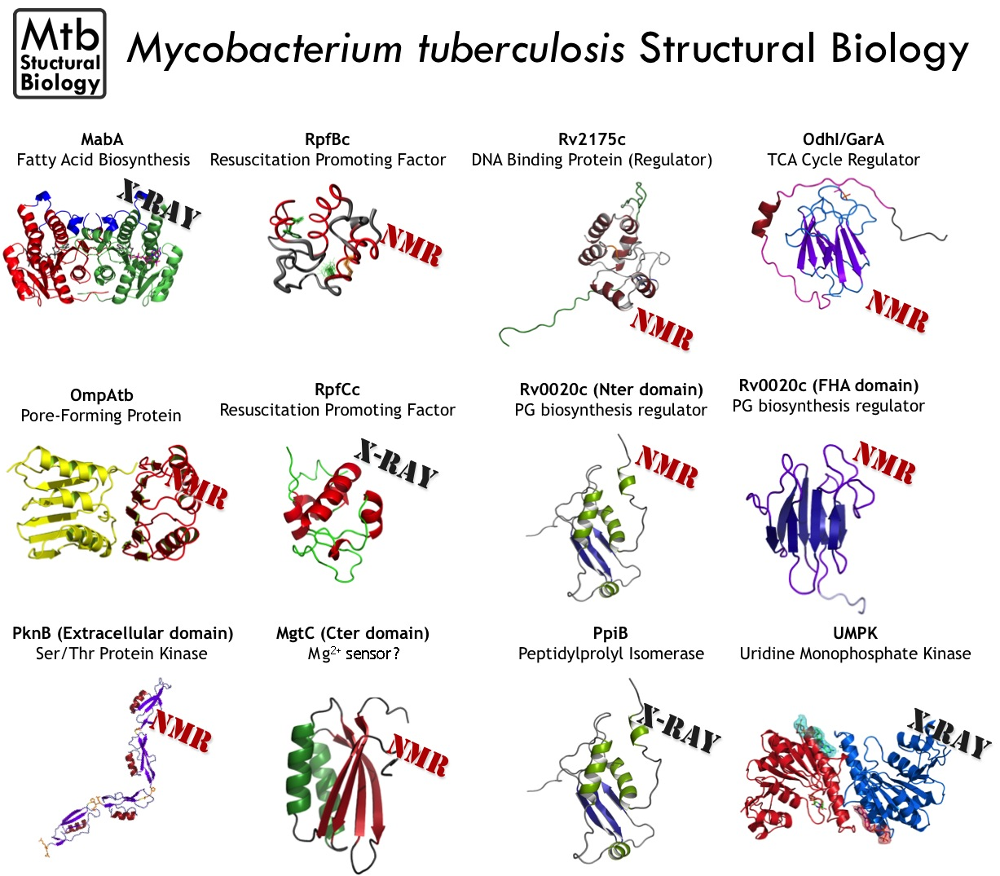
Regulation via Ser/Thr Protein Kinases (STPK) has emerged as an essential mechanism in controlling key pathways in the bacillus (metabolism, cell division, expression regulation). Despite the fact that the STPKs have been extensively studied, the molecular mechanisms of its phosphoregulation were poorly understood. In collaboration with different laboratories we engaged studies to understand the activity modulation of STPKs substrates to unravel new regulation pathways within the bacteria. We solved the structure of the unphosphorylated and phosphorylated isoforms of the OdhI protein (also named GarA) in solution, a central regulator of the tricarboxylic acid (TCA). We discovered a major conformational changes upon phosphorylation of a disordered region of the protein (Barthe 2009). We studied a FHA modular protein called Rv0020c. We solved the structure of its domains by NMR and studied their interaction with STPK demonstrating a fine tuning of the STPK/Rv0020c interaction via the phosphorylation of an unstructured STPK domain (Roumestand 2011). Furthermore, we solved the structure of a transcriptional regulator in solution, and revealed a DNA binding regulation mediated by STPK phosphorylation (Cohen-Gonsaud 2009). Other studies in M.tuberculosis focussed on the STPK regulation of the essential mycolic acid biosynthesis (Veyron-Churley 2012), the S-Adenosylhomocysteine hydrolase activity (Corrales 2013), and the CcpA regulation in S. aureus (Leiba 2012).
One of the keys of Mycobacterium tuberculosis’ success as a pathogen is its ability to persist in the host organism in a latent state for years after the first phase of infection. We have been interested for long time in the exit mechanism of latency (Cohen-Gonsaud 2005). The STPK PknB has been proposed to be the receptor for a dormancy exit signal. With proven combined experience in the field, our team and Galiana Mukamolova’s group (University of Leicester) initiated a study to isolate and study the potential molecular determinant of resuscitation in M. tuberculosis. We first solved the structure of the external domain of the kinase in solution that revealed an original fold (Barthe 2010). After extensive studies we can now propose a model for the PknB related resuscitation mechanism (manuscript in preparation).
Persistence and Virulence
Collaboration with : A. Blanc-Potard (DIMNP – Montpellier ; K. Brodolin (CPBS, Montpellier), L. Brodin (Institut Pasteur, Lille), G. Mukamolova (University of Leicester)
One of the main projects over the last years has been related to the mechanism that has been unravelled by our collaborator Galiana Mukamolova at the University of Leicester. They demonstrated that three of the main TB antibiotics have the ability to enhance the survival of the bacillus during the stationary phase. Combining molecular modelling, biochemical, genetic, transcriptomic, and animal studies we proposed a complete model to explain the observed phenomenon (Turapov submitted). Another important project has been initiated in mid-2012 in collaboration with the group of Pricille Brodin (Institut Pasteur Lille). The ability of the bacillus to arrest phagosome maturation is a major mechanism that allows its survival within host macrophages. To identify mycobacterial genes involved in this process, they developed a high-throughput phenotypic cell-based assay enabling individual sub-cellular analysis of over 11,000 M.tuberculosis mutants. Among them two essential protein were isolated.
Biologie structurale d’Apicomplexa (CR)
Collaboration with : S. Delbeq (University of Montpellier), T. Schetters (Intervet International B.V. – The Netherlands), H.Vial (University of Montpellier 2)
The structural study of surface antigens from the merozoite membrane of Babesia is still in progress and constitutes a fruitful collaboration with the team of S. Delbecq (Faculty of Pharmacy, Montpellier) and Intervet® (MSD), one of the leader companies in veterinary vaccines. A new collaboration has been initiated with the group of H. Vial (CNRS UMR 5235, Montpellier) to tackle the malaria parasite Plasmodium falciparum, an Apicomplexa responsible for a toll of one million human deaths per year, with almost half of the human population at risk of contracting the disease. Our objectives are i) to determine the 3D structures of the catalytic domains of PfCCT, an enzyme involved in the metabolism of phospholipids in the parasite, and ii) to study the interaction of the catalytic domains with their own substrates and with compounds inhibiting PC biosynthesis These compounds are mono- and bis-thiazoliums developed in Dr. H. Vial laboratory and are currently under clinical development (Sanofi-Aventis).

