Link to publication: https://www.nature.com/articles/s41594-023-00920-0
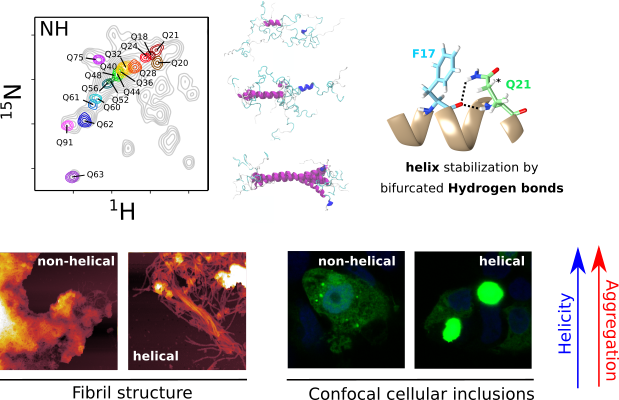
Huntington’s Disease (HD) is a deadly neurodegenerative disorder caused by an expansion of the CAG codons in the first exon of the HTT gene that, after translation, results in an extended poly-glutamine (poly-Q) tract in the N-terminal region of the protein huntingtin (httex1). Only individuals with more than 35 consecutive glutamines in this track develop HD. The structural changes occurring to httex1 when increasing its length beyond this pathological threshold remain poorly understood, precluding a molecular understanding of the pathology. The high-resolution structural investigation of httex1 had been considered as impossible due to its intrinsic flexibility, which preclude the use of X-ray crystallography and cryo-Electron microscopy, and the strong compositional bias that hampers the use of traditional NMR approaches. During the last years, our group has developed chemical biology approaches to incorporate isotopically labelled amino acids into proteins in a site-specific manner, enabling the high-resolution NMR investigation of highly repetitive proteins, such as httex1.
The systematic application of site-specific isotopic labelling has enabled the residue-specific NMR structural characterization of poly-Q tracts for pathogenic (Q46 and Q66) versions of httex1. These NMR data have been integrated with SAXS and computational approaches, to derive ensemble models and define the rules governing the structure of the poly-Q. Our analysis reveals that the poly-Q tract adopts long α-helical conformations propagated and stabilized by glutamine side chain to backbone hydrogen bonds, and highlights the relevance of the flanking regions in defining this structure. From a biomedical perspective, we show that α-helical stability is a stronger signature than the number of glutamines in defining aggregation kinetics, both in vitro and in cells, and the structure of the resulting fibrils. Our observations provide a structural perspective of the pathogenicity of expanded httex1 and pave the way to a deeper understanding of poly-Q-related diseases.