Conformation of a protein in solution
| Service |
Study of the conformation of a protein in solution
|
|
| Goal |
Determination of the conformation and the folding of a protein sample in solution |
|
|
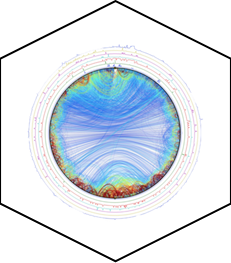
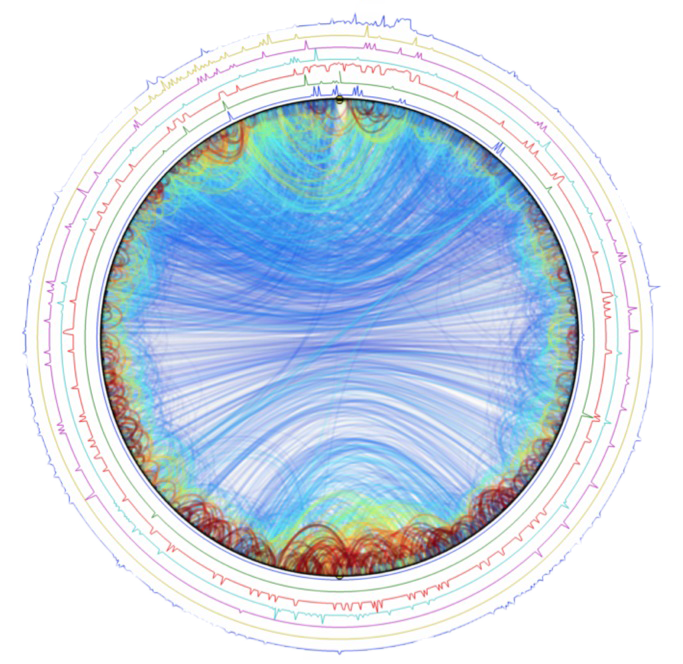
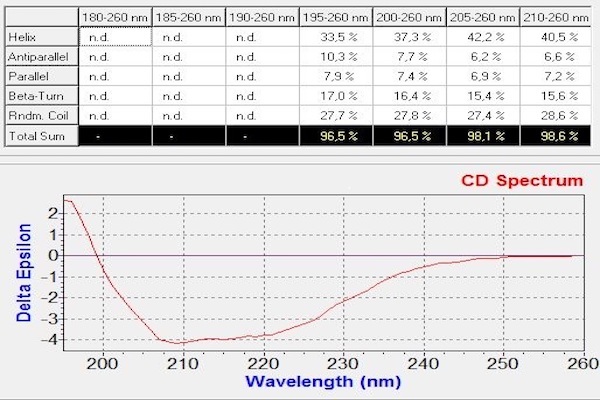
Circular dichroism is a non-destructive spectroscopic method. It is based on the ability of a sample with a chiral chromophore and placed in an asymmetric environment to absorb circularly right polarized light and circularly left polarized light differently. Thus, this method is applicable to protein. The dichroic spectrum corresponds to the difference in absorbance between these two types of light, for each wavelength. In the case of proteins, the measurement of circular dichroism in far UV (180-260 nm, absorption zone of the peptide bond) contains information on their secondary structure (α helices, β sheets, turn and random coil). Data deconvolution allows an estimation of respective proportions of the different secondary structure elements present in the protein.
|
||
|
Example of spectrum obtained on a protein of interest with CD Chirascan and the corresponding deconvolution giving the percentage of each secondary structure elements in the protein. |
||
| Equipement |
Circular Dichroism
|
||||
 |