• Structure and Activation of G-Protein Coupled Receptors •
Coordinator: Hélène Déméné
NMR Staff : Hélène Déméné, Yang Yinshan
This work is issued from a close collaboration between the CBS partners and the IGF partners.
G-Protein Coupled Receptors represent the largest class of membrane surface cell receptors involved in signal transduction in eukaryotes. They sense molecules outside the cell and activate signal transduction pathways inside the cell and, ultimately, cellular responses, by coupling with G-proteins. Our research themes focus on the receptors of the vasopressin (V1R and V2R) and on the µ-opioid receptor (µOR).
In the past, we have deciphered the structural features of isolated intracellular loops of V1aR and V2R. We have shown how they fold by themselves, independently from the rest of the receptors, and are also able to recruit intracellular effectors by themselves. We have proposed a method to produce them under a recombinant form mimicking the natural anchoring in the receptor.
We now investigate on the structure-activity of whole GPCRs, taking advantage of the sensitivity of methyl-based NMR. In particular, we have shown that the ligand- and G-protein binding interfaces are weakly coupled in µOR, extending the concept observed before for the ß2 adrenergic receptor by the Kobilka's team. Based on the NMR spectral parameter changes upon interaction of µOR with various agonists as well as with a G-protein mimetic, we could propose a model of event propagation during activation.
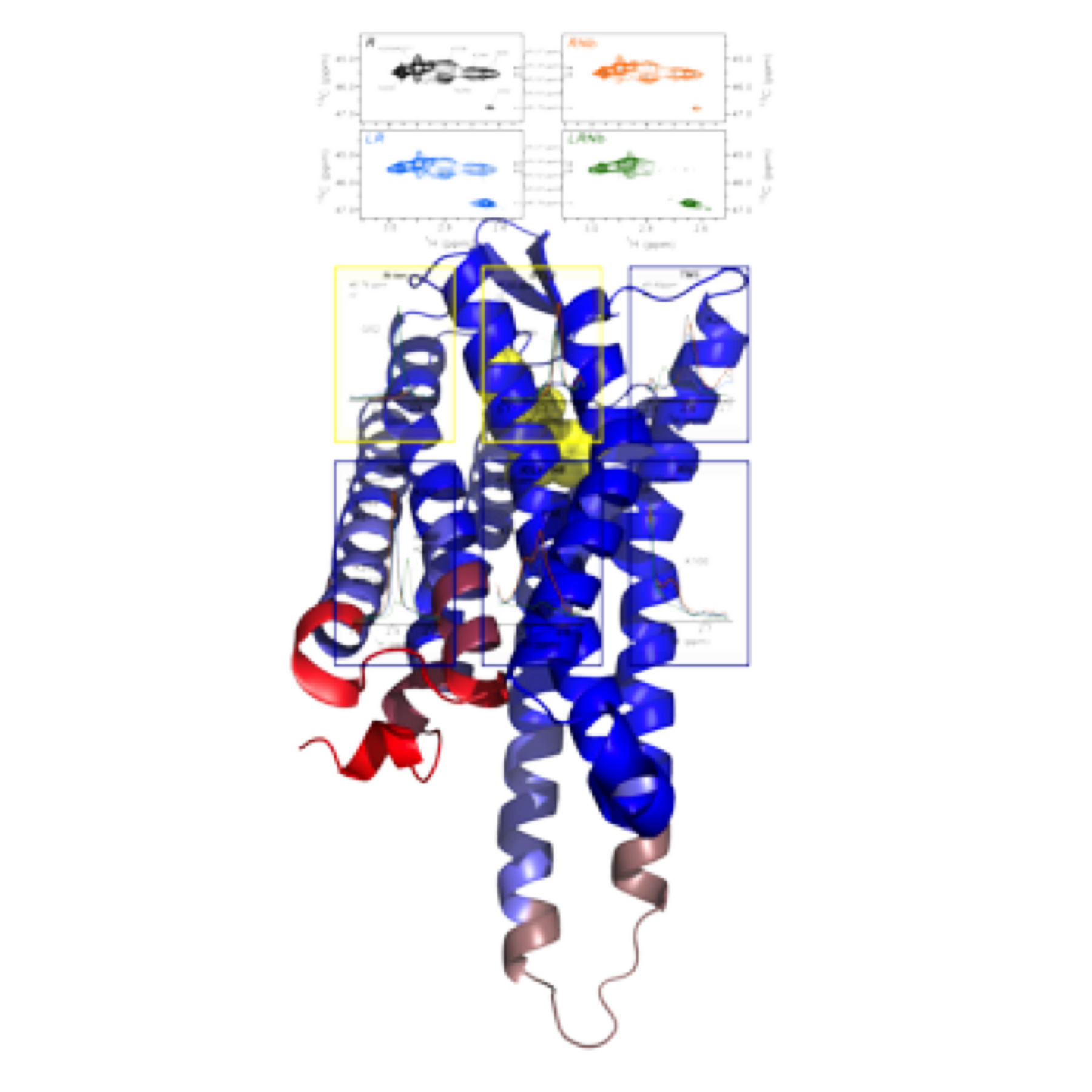 |
Figure: agonist binding promotes primary structural changes in the first intracellular loop and the H8 helix, whereas conformational adaption in TM5 and TM6 is limited compared to the full-active state obtained in presence of agonist and G-protein. |
Coll. Sébastien Granier, (IGF); Christiane Mendre, (IGF); Bernard Mouillac, (IGF)

