Mechanistic and structural bases of recognition between avirulence proteins (AVR) and resistance proteins (R).
André Padilla & Karine de Guillen

The molecular bases of plant resistance against pathogenic fungi aggressions are poorly understood. This immunity is based on two levels defence system. The first line involves the detection of microbial molecules, such as flagellin or components of the cell wall of the pathogenic microorganism, leading to resistance. To avoid this first level the fungus secretes, during infection, small proteins called avirulence (AVR), which are considered as key elements of fungal pathogenicity but whose functions are largely unknown. The recognition of these plants pathogenic effectors is triggered by resistance proteins (R), which constitute the second layer of defence. This recognition leads to a "hypersensitive" response, which is characterized by localized cell death, avoiding plant colonization by the pathogen.
The fungus Magnaporthe oryzae is the major pathogen of rice. The disease triggered by this fungus is called rice blast. It is responsible for large economic losses and is a major threat to food security. In recent years collaboration with the team of T. Kroj (INRA-Montpellier BGPI) was established. Our collaborators have demonstrated the interaction between avirulence protein of M.oryzae AVR-Pia and AVR-CO39 and resistance proteins RGA4-RGA5 (Cesari et al, Plant Cell, 2013, Ribot et al, The Plant Journal 2013). The production of recombinant avirulences proteins allowed us to determine their three-dimensional structures by NMR. These recent findings raise several questions:
- How are AVR proteins specifically recognized by "their" R protein?
- What are the key amino acids involved in the interaction?
- How is AVR recognition linked to defence activation in R proteins hetero pair?
Validation of structures (AVR and R-domains) by functional studies will help us to understand the mechanisms of recognition and co-evolutions between AVR and R
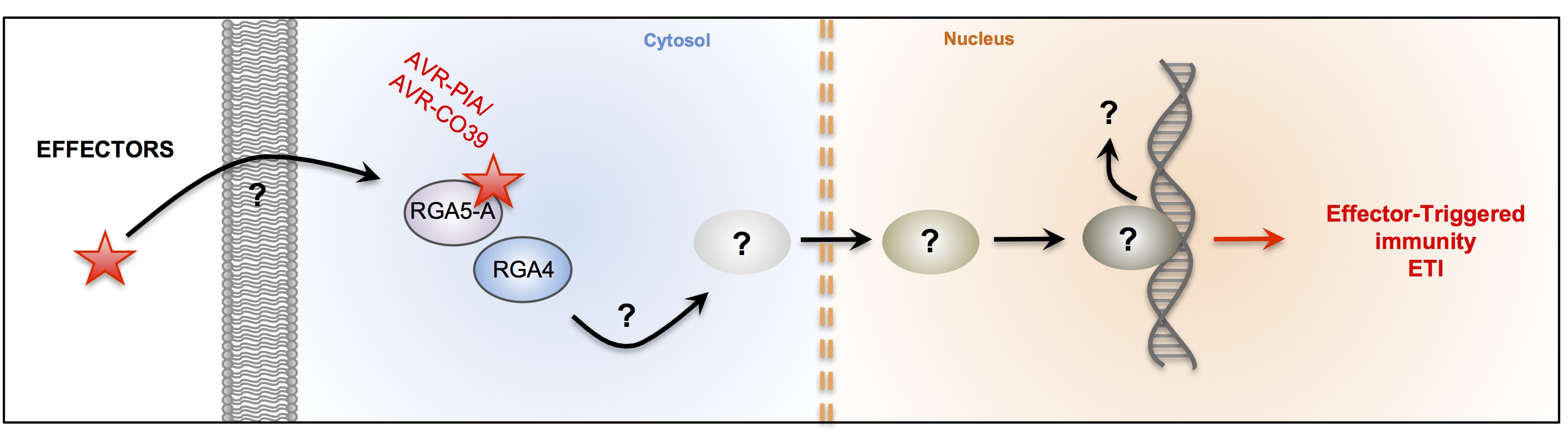
Interaction between fungus Avr proteins and plant R proteins. (adapted from Liu et al 2014)
External collaboration: T. Kroj INRA-BGPI – Montpellier – France
Structure and dynamics of RYMV viral encoded P1 protein
Hélène Déméné & Yinshan Yang
The P1 protein is a crucial protein of the RYMV (Rice Mottle Yellow) virus that infects the most productive rice plants in Africa. Using an integrative approach combining X-Ray and NMR spectroscopy at the CBS, we have characterized the structure and revealed the dynamics of the RYMV P1 protein that are linked to the mode of viral activation.
External collaboration: Florence Vignols and Christophe Brugidou (IRD)
Structure of Major Antigens from Apicomplexa.
Christian Roumestand & Yinshan Yang.
Babesiosis (formerly known as piroplasmosis) is a tick-borne disease caused by the intraerythrocytic development of protozoa parasites from the genus Babesia. Like Plasmodium falciparum, the agent of Malaria, or Toxoplasma gondii, responsible for human toxoplasmosis, Babesia belongs to the Apicomplexa family. Babesia canis is the agent of the canine babesiosis, while Babesia divergens infects essentially bovine. The identification and characterization of parasite surface proteins represent major goal, both to understand the molecular bases of Apicomplexa invasion process and for the potential as vaccines of such antigens. Indeed, it has been shown that the GPI membrane anchored protein Bd37, a 37 kDa antigenic adhesion protein from B. divergens, was able to induce complete protection against various parasite strains. Its orthologue in B. canis, Bc28.1, has been described as a 28 kDa membrane protein GPI anchored at the surface of the merozoite. We have defined the erythrocyte binding function of these two proteins and determined their high-resolution solution structure using NMR spectroscopy (Delbecq et al., 2008: Yang et al., 2012). Surprisingly, although these proteins are thought to play a similar role in the adhesion process, the structure of Bc28.1 (see Figure) appears unrelated to the structure of Bd37. Site-directed mutagenesis experiments also suggest that the mechanism of the interaction with erythrocyte membrane could be different for the two proteins. The resolution of the structure of these adhesion proteins represents a milestone for the characterization of the parasite erythrocyte binding and its interaction with the host immune system.
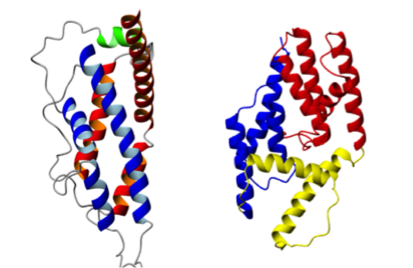
Solution structure of adhesion proteins from Babesia.
(Left) Bd37 from B. divergens; (Right) Bc28 from B. canis
External Collaborations:
· R. Cerdan – DIMNP – Montpellier – France
· S. Delbecq – University Montpellier I – Montpellier – France

