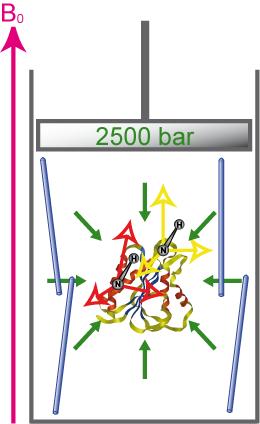
RDC under pressure (Nathalie Sibille)
Collaboration : Christian Roumestand and Cathy Royer, CBS, Montpellier
Accurate description of protein folding and stability, in normal and pathological conditions, has been a major area of biophysical chemistry for over 50 years. Although much progress has been made in recent years to achieve this objective, a number of important questions remain. The physical basis of pressure effects on the structure and stability of the protein remained a mystery unlike the effects of temperature.
We have recently characterized several alignment media that are amenable to high-pressure. This enables the measurement of RDCs in a broad range of pressures that will enable the structural characterization of protein states emerging under these conditions. For more details see :
Sibille N*, Delarole M, Royer C, Roumestand C. Measuring Residual Dipolar Couplings at High Hydrostatic Pressure : Robustness of Alignment Media to High Pressure. J Biomol NMR. (2014).
Advanced Computational Approaches to study protein motions. (Pau Bernadó)
Collaboration : Juan Cortés (LAAS – Toulouse)
The study of flexible systems at atomic level requires detailed description of the structure and the mechanisms inducing their fluctuations. In that context, our group is interested in advanced computational approaches allowing the exploration of the conformational space in an efficient manner to study mobility in broad time-scales. We are interested in Normal Mode approaches that perturb 3D structures in a physically meaningful manner. Derived conformations can be evaluated using SAXS data, which are exquisitely sensitive to large conformational fluctuations.
Robotics-inspired algorithms have been demonstrated to be very efficient in sampling the 3D space available to macromolecules. In collaboration with Juan Cortés (LAAS-Toulouse) we aim to apply these approaches to study conformational transitions in IDPs.

