Molecular mechanisms of functional disordered C-terminal regions of GPCRs and impact on arrestin signaling pathways (PI: Nathalie Sibille)
People Involved: N. Sibille, P. Bernadó, F. Allemand, A. Fournet, A. Thureau, A. Mouhand, M. Guillien
This project aims to elucidate the details and principles of non-G protein dependent signaling. Arrestin-mediated and ligand-induced biased signaling is a hot topic currently in GPCR and drug research in general. By focusing on ghrelin, b2ar and V2 receptors, we have chosen both varied GPCRs to investigate by state-of-the-art biochemical and especially biophysical methods the func- tional disordered regions of GPCRs with regard to their interaction with arrestins.
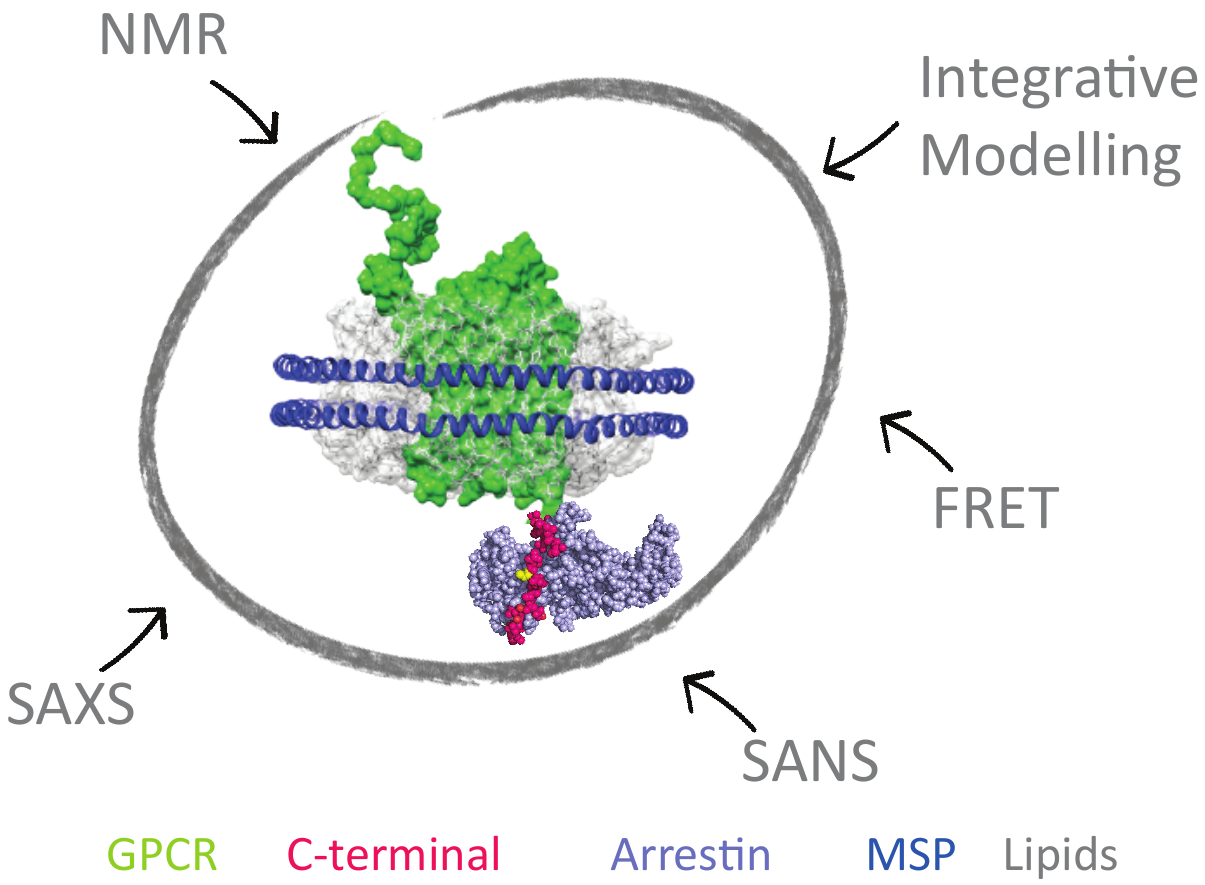
To design more effective drugs without side effects, it is essential to better understand the molecular mechanism of GPCR (G-Protein Coupled Receptors), which are targeted by one third of drugs on the market. Some crucial cell signaling pathways are mediated by the interaction of the cytoplasmic C-ter- minal part of the GPCR with β-arrestin. This functional interaction is modulated by GPCR-associated kinases (GRK). This project aims at revealing the link between C-tail phosphorylation patterns by the various GRKs, their structural dynamics and the different related arrestin «func- tional conformations». This will be achieved by combining solution spectroscopic techniques, such as NMR and SAS, on model systems of increasing complexity ranging from isolated peptides to purified signaling complexes into membrane-mi- micking systems (nanodiscs). This study will reveal the struc- tural and dynamic mechanisms necessary for the interaction of the C-terminal regions of GPCRs with β-arrestin and provide essential information to guide the rational design of peptide mimetics able to modulate specific signaling cascades.
Main collaborators: J.L. Banères (IBMM, Montpellier), B. Mouillac (IGF, Montpellier), L. Arleth (U. Copenhagen)
Grant: ANR GPCteR