A le Maire, W Bourguet
NRs are important for homeostatic control and for adaptation to environmental variation. They thus participate in molecular mechanisms whose dysregulation is associated with proliferative, reproductive, and metabolic diseases, such as cancer, infertility, obesity, or diabetes. As such NRs are the molecular target of approximately 15% of authorized medications.
We have examined multiple medically important NR/corepressor aberrant interactions with the objective of designing small molecules or peptidomimetics that could specifically disrupt the undesired interactions. In collaboration with M Privalsky, we described the specific interaction surfaces between pathogenic thyroid hormone receptor β (TRβ) mutants and corepressors, and demonstrated that a ligand (TRIAC) is a potential therapeutic agent for patients suffering from resistance to thyroid hormone (RTHβ) syndromes [Harrus, Thyroid, 2018]. As part of an ANR project (ThyroMUT2 2015-2019) conducted in collaboration with F Flamant and clinicians, we are currently finalizing a similar work on a set of recently described TRα mutants causing a new type of RTHα where we decipher the molecular basis underlying the phenotype variability associated with different THRα mutations [Espiard, J Clin Endocrinol Metab, 2015 ; le Maire, Thyroid, 2020].
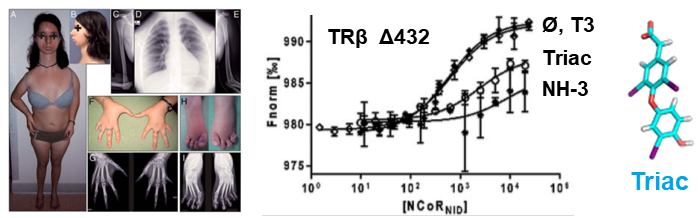
The resistance to thyroid hormone (RTH) syndrome results from the incapacity of corepressors to dissociate from TR upon natural hormone (T3) binding.
As a main player in drug resistance, the pregnane X receptor (PXR) has also been (and remains) the subject of intense research in our group. Upon activation by many external compounds, including drugs, PXR induces the transcription of genes encoding xenobiotic metabolizing enzymes and efflux pumps that trigger body detoxification. However, this process severely reduces the effectiveness of drugs by shortening their lifetime. In a collaborative work with Team 4, we solved the crystal structure of PXR in complex with an anticancer drug that was recently shown by our collaborator P Balaguer to act as a potent PXR agonist (EC50 < 100 nM). We then rationally designed derivatives that have lost their binding capacity to PXR while retaining high binding affinity for their pharmacological target (work in progress). Another way to prevent premature metabolization of drugs is to inhibit PXR activity. In collaboration with JM Pascussi, M Amblard we rationally designed a bi-functional ligand also referred to as PROTAC (PROteolysis-TArgeting Chimera) that simultaneously binds to PXR and recruits E3 ligases to direct PXR to degradation by the proteasome (ongoing work).
The peroxisome proliferator-activated receptor γ (PPARγ) is a master regulator of adipogenesis and a potent modulator of whole-body lipid metabolism and insulin-sensitivity, making it a valuable pharmaceutical target against obesity and diabetes. Interestingly, it has been recently reported that phosphorylation of PPARγ on S273 correlates with repression of a subset of target genes shown to be dysregulated in obesity. In the aim of identifying novel PPARγ ligands with therapeutic potential, we have described the interaction surface between PPARγ and CDK5 and developed an assay pipeline capable of detecting ligands that physically bind to PPARγ and inhibit the CDK5-mediated phosphorylation without invoking its transcriptional activity and the associated side effects [Ribeiro, Front Endocrinol, 2018 ; Ribeiro, J Struct Biol, 2019; Dias, Front Endocrinol, 2020] (coll. AC Figueira).
Ivermectin (IVM) is the most important broad spectrum antiparasitic drugs used today. Unfortunately, resistance to IVM has appeared in parasites and it seriously jeopardizes the success of current parasite control. By using an original IVM-resistant C. elegans strain, our collaborator A. Lespine showed that the Nuclear Hormone Receptor NHR-8 is a key player in the dynamic development of resistance to IVM. NHR-8 stands at the cross-road of major metabolic pathways in nematodes. We propose to solve NHR-8 structure and to identify its ligands to help deciphering NHR-8 functions and translateing these insights to other target parasitic nematodes with the goal of developing new anti-infectious approaches.
Main collaborators: F Flamant (IGFL Lyon), M Privalsky (UC Davis USA), AC Figueira (CNPEM Brasil), JM Pascussi (IGF Montpellier), M Amblard (IBMM Montpellier), P Balaguer (IRCM Montpellier), A. Lespine (INRAE, Toulouse), Team 4 (CBS)

